Reconstructing neural circuits following brain and spinal cord injury
CNS injuries due to stroke or trauma disrupt the neural circuits and result in severe functional deficits. The brain and spinal cord have very limited capacity to reconstruct the circuits once they are damaged, and therefore no effective therapeutic methods have been developed so far. We previously demonstrated that spared motor and autonomic circuits are dynamically reorganized after injuries and influence the recovery process of functions (Ueno et al., Nat Neurosci 2016, Brain 2012). These results suggest that controlling the rewiring of circuits could help establish proper connections and support functional recovery.
The goal of our study is to understand the process of rewiring and the underlying molecular mechanisms to achieve functional recovery. We study the process of reconstruction of neural connections at the local circuit to system level in the brain and further the ceullular and molecular mechanisms that drive the rewiring process through the responses and interactions of neuronal and glial cell subtypes (Nakamura et al., J Neurosci 2021; Sato et al., Front Neurosci 2021; Inoue et al., Front Neural Circuits 2025; Sato et al., bioRxiv 2025).
To this end, we are analyzing neural systems of both normal and injured brain and spinal cord, using cutting-edge techniques including mouse genetics, viral tracers, optogenetics, chemogenetics, omics analyses, and 3D behavior analysis. Our sudy will elucidate the overall picture of the process and mechanism of neural circuit repair in multi-layered levels. We believe that this study will pave the way to develop novel strategies to repair the circuits and restore neural functions.
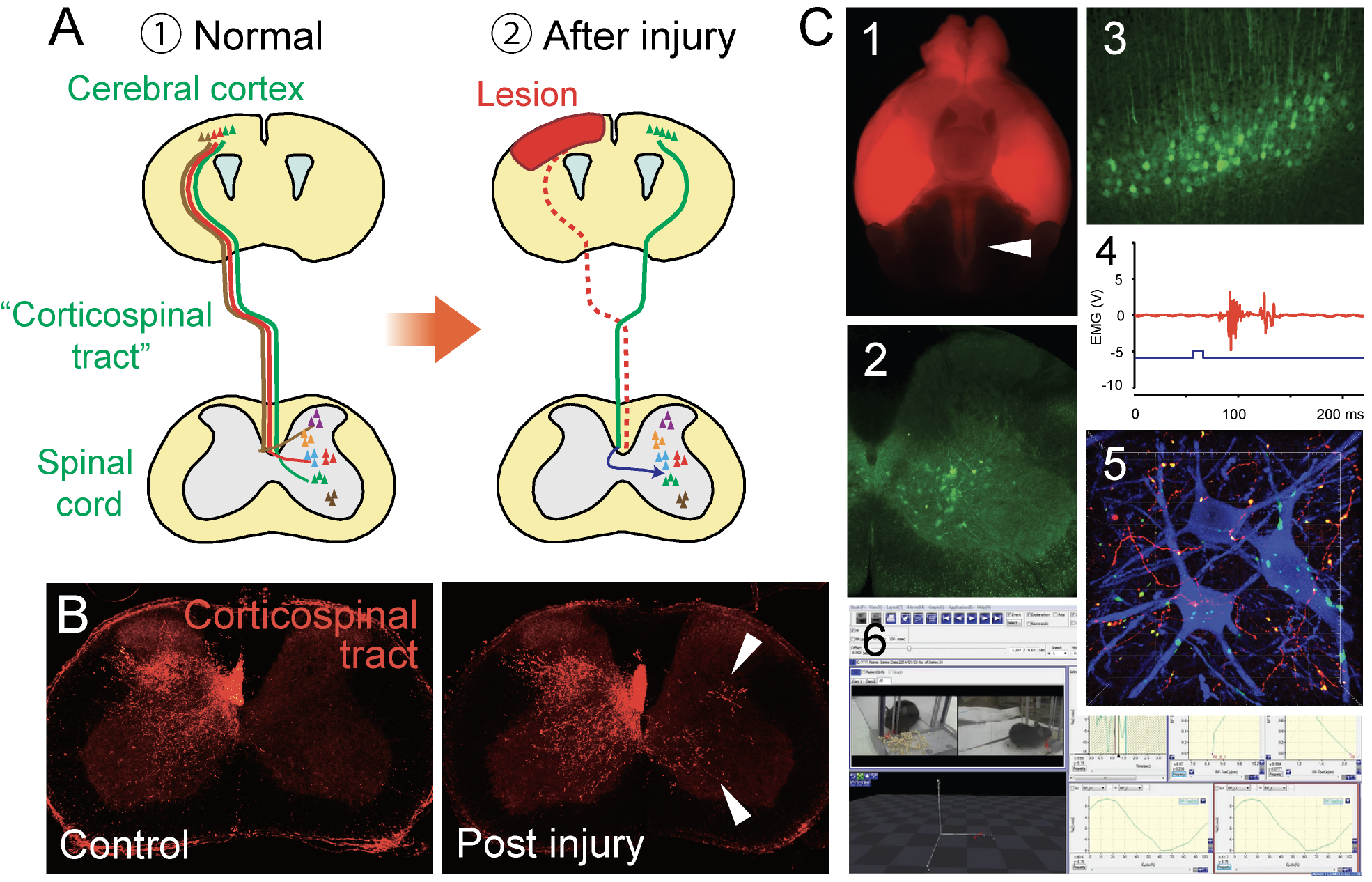
Motor circuits and their rewiring following brain / spinal cord injury.
(A) We are targeting the "corticospinal tract (CST)", an essential neural circuit for skilled and voluntary movement. The circuit is rewired following the injury (a blue arrow). (B) Axon sprouting of the CST, labeled with anterograde neural tracers (arrowheads, modified from Ueno et al., Brain (2012)). (C) We are using a variety of tools to analyze neural circuits. Labeling the CST (1) and spinal interneurons (2) by mouse genetics; labeling of neurons by transsynaptic viral tracers (3); electrical responses of muscles by optogenetic approach (4); connection of CST axons and spinal interneurons (5); 3 dimensional kinematic analyses of skilled behaviors (6) (modified from Ueno et al., Cell Rep (2018)).
Selected papers
Corticospinal circuits from the sensory and motor cortices differentially regulate skilled movements through distinct spinal interneurons.
*Ueno M, Nakamura Y, Li J, Gu Z, Niehaus J, Maezawa M, Crone SA, Goulding M, Baccei ML, Yoshida Y.
Cell Rep 23: 1286-1300, 2018
https://www.sciencedirect.com/science/article/pii/S2211124718305254?via%3Dihub
Press release from
 Niigata University
Niigata University
Introduced in Niigata Nippo (May 15, 2018) and the Science News (May 25, 2018).
Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury.
Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG, Yoshida Y.
Nat Neurosci. 19(6):784-7, 2016
Press release from
 JST
JST
 Cincinnati Children's Hospital
Cincinnati Children's Hospital
 Ohio State Univ
Ohio State Univ
Introduced in Jikken Igaku and Japan Spinal Cord Foundation news
Layer V cortical neurons require microglial support for survival during postnatal development.
*Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T.
Nat Neurosci. 16(5): 543-551, 2013
Highly Cited Paper in Web of Science (Thomson Reuters)
Intraspinal rewiring of the corticospinal tract requires target-derived BDNF and compensates lost function after brain injury.
Ueno M, Hayano Y, Nakagawa, H, Yamashita T.
Brain.135(4): 1253-67, 2012
Introduced in NHK TV news "Ohayo Kansai" (April 3, 2012)
Introduced in Jiji Press (April 2, 2012), Yomiuri shimbun (April 4, 2012)
Our studies are supported by
Challenging Research (Pioneering)(JSPS: KAKENHI) (2017–19)
Grant-in-Aid for Young Scientists (A) (JSPS: KAKENHI) (2017–20)
Grant-in-Aid for Scientific Research on Innovative Areas (MEXT: Adaptive Circuit Shift) (2017–18) Link
Senri Life Science Foundation (2017)
Takeda Science Foundation (2017)
Narishige Neuroscience Research Foundation (2017)
Ube Industries Foundation (2017)
Kato Memorial Bioscience Foundation (2017)
Japan Heart Foundation (2017)
Tokyo Biochemical Research Foundation (2017)
PRESTO (JST) (2013–17) Link
Postdoctral Fellowship for Research Abroad (JSPS) (2012–13)
Kanae Foundation for the Promotion of Medical Science (2012)
Grant-in-Aid for Young Scientists (B) (MEXT: KAKENHI) (2009–11)
The goal of our study is to understand the process of rewiring and the underlying molecular mechanisms to achieve functional recovery. We study the process of reconstruction of neural connections at the local circuit to system level in the brain and further the ceullular and molecular mechanisms that drive the rewiring process through the responses and interactions of neuronal and glial cell subtypes (Nakamura et al., J Neurosci 2021; Sato et al., Front Neurosci 2021; Inoue et al., Front Neural Circuits 2025; Sato et al., bioRxiv 2025).
To this end, we are analyzing neural systems of both normal and injured brain and spinal cord, using cutting-edge techniques including mouse genetics, viral tracers, optogenetics, chemogenetics, omics analyses, and 3D behavior analysis. Our sudy will elucidate the overall picture of the process and mechanism of neural circuit repair in multi-layered levels. We believe that this study will pave the way to develop novel strategies to repair the circuits and restore neural functions.

Motor circuits and their rewiring following brain / spinal cord injury.
(A) We are targeting the "corticospinal tract (CST)", an essential neural circuit for skilled and voluntary movement. The circuit is rewired following the injury (a blue arrow). (B) Axon sprouting of the CST, labeled with anterograde neural tracers (arrowheads, modified from Ueno et al., Brain (2012)). (C) We are using a variety of tools to analyze neural circuits. Labeling the CST (1) and spinal interneurons (2) by mouse genetics; labeling of neurons by transsynaptic viral tracers (3); electrical responses of muscles by optogenetic approach (4); connection of CST axons and spinal interneurons (5); 3 dimensional kinematic analyses of skilled behaviors (6) (modified from Ueno et al., Cell Rep (2018)).
Selected papers
Corticospinal circuits from the sensory and motor cortices differentially regulate skilled movements through distinct spinal interneurons.
*Ueno M, Nakamura Y, Li J, Gu Z, Niehaus J, Maezawa M, Crone SA, Goulding M, Baccei ML, Yoshida Y.
Cell Rep 23: 1286-1300, 2018
https://www.sciencedirect.com/science/article/pii/S2211124718305254?via%3Dihub
Press release from

Introduced in Niigata Nippo (May 15, 2018) and the Science News (May 25, 2018).
Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury.
Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG, Yoshida Y.
Nat Neurosci. 19(6):784-7, 2016
Press release from



Introduced in Jikken Igaku and Japan Spinal Cord Foundation news
Layer V cortical neurons require microglial support for survival during postnatal development.
*Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T.
Nat Neurosci. 16(5): 543-551, 2013
Highly Cited Paper in Web of Science (Thomson Reuters)
Selected in
 F1000Prime
F1000Prime

Introduced in
 Life Science Review
Life Science Review

Press release from

JST / Osaka Univ

Intraspinal rewiring of the corticospinal tract requires target-derived BDNF and compensates lost function after brain injury.
Ueno M, Hayano Y, Nakagawa, H, Yamashita T.
Brain.135(4): 1253-67, 2012
Introduced in NHK TV news "Ohayo Kansai" (April 3, 2012)
Introduced in Jiji Press (April 2, 2012), Yomiuri shimbun (April 4, 2012)
Press release from
 Osaka Univ
Osaka Univ

Introduced in
 Brain Blogger
Brain Blogger

Our studies are supported by
Challenging Research (Pioneering)(JSPS: KAKENHI) (2017–19)
Grant-in-Aid for Young Scientists (A) (JSPS: KAKENHI) (2017–20)
Grant-in-Aid for Scientific Research on Innovative Areas (MEXT: Adaptive Circuit Shift) (2017–18) Link
Senri Life Science Foundation (2017)
Takeda Science Foundation (2017)
Narishige Neuroscience Research Foundation (2017)
Ube Industries Foundation (2017)
Kato Memorial Bioscience Foundation (2017)
Japan Heart Foundation (2017)
Tokyo Biochemical Research Foundation (2017)
PRESTO (JST) (2013–17) Link
Postdoctral Fellowship for Research Abroad (JSPS) (2012–13)
Kanae Foundation for the Promotion of Medical Science (2012)
Grant-in-Aid for Young Scientists (B) (MEXT: KAKENHI) (2009–11)

